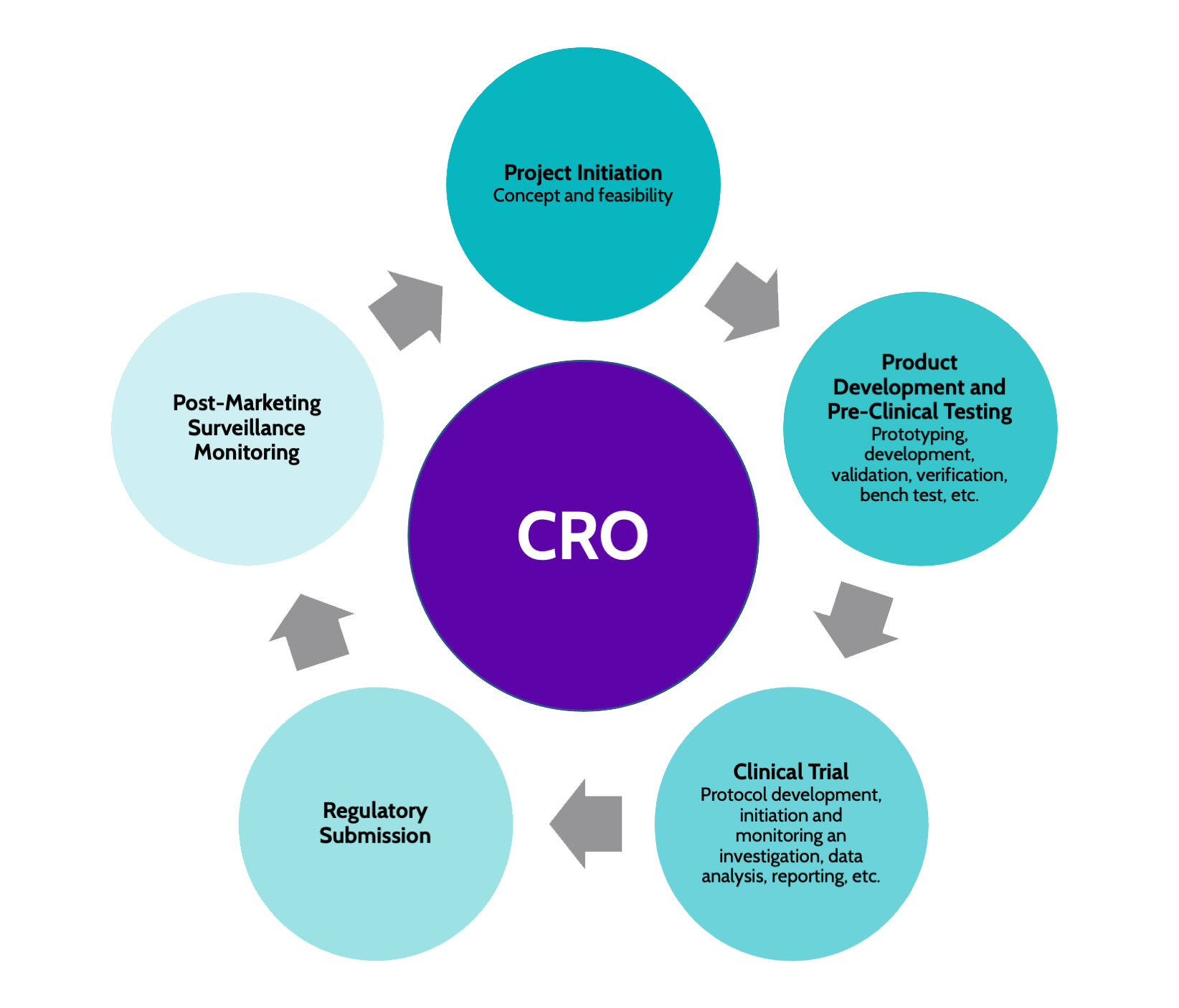
Contract Research Organization (CRO)
Moving your Research to the NEXTSTAGE
High-quality, well-prepared data might mean the difference between a successful regulatory submission and a frustratingly long approval process. A boutique, specialty-focused CRO like NextStage brings experience, commercially-centric perspective, and quality to data collecting, statistical analysis, and clinical trial reporting, whereas a full-service CRO may consider data as an afterthought. Trust the clinical data professionals with your data from research design to final submission.
Clinical Data Management
The process of gathering, validating, and preparing data for statistical analysis in clinical trials is known as clinical data management (CDM). To gather and preserve data, you must first set up a clinical data collecting tool(s) or a clinical database based on the study protocol. Following the storage of data, it is critical to undertake data validation methods to guarantee that accurate data is obtained and that all applicable global and local regulatory standards are met. When the data is ready for analysis, the database is locked to prevent tampering, and the datasets are transferred to the statistics and programming teams for analysis.
Services:
- Medical Coding setup and implementation
- CRF/E-CRF design and maintenance
- Electronic data integration
- E-PRO/E-COA & E-Source
- Data management plan (DMP) and related documents creation
- Database locks & post lock archival
- Data query generation and maintenance
- SAS listing development and more…
Biostatistics
To establish the efficacy and effectiveness in clinical trials, biostatistics is required for any experimental medication or device development programs. This comprises study designs, study conduct, determining the most efficient data collection sites, and determining how analysis and reporting should be done.
Services:
Biostatistical consulting for protocol design, sample size calculation, power calculations, and/or study randomization production, as well as study level analysis of Tables, Listings, and Figures.
- Study report outputs production
- Creating Statistical Analysis Plans (SAP)
- Interim Analysis/Data Safety Monitoring Boards
- PK/PD Analysis
- CDISC
- Clinical Study Reports
- Meta-analysis
- Production of statistical report/clinical study report and more…
Statistical Programming
Clinical trial reporting is made possible by statistical programming, which allows for the generation of regulatory-ready, analyzed datasets and the creation of Tables, Listings, and Figures (TLFs). These deliverables assist you in comprehending the safety and efficacy of your experimental product, as well as the results of your trial hypotheses.
Services:
- Interim Analysis Support
- CDISC mapping/conversion capabilities
- Analysis Datasets and TLFs Development, and QC for standard studies and Integrated Summaries
- SAS and R Programming
- Macro production, validation and optimization
- Annual Clinical Trial Safety Updates
- CDISC ADaM submission-ready Dataset Development and QC for Submission Compliance
- Analysis Datasets and TLFs Development and more…
Medical Writing
From the pharmaceutical sector to clinical research companies to academia, we have a wide range of knowledge and experience. NextStage’s documents go through a rigorous scientific, statistical, editorial, and quality control review process.
Services:
- Study Protocols
- Patient Safety Narratives
- Investigator Brochures
- Pharmacovigilance documents such as Periodic Safety Update Reports
- ICH GCP compliant Clinical Study Reports (CSRs)
- Standard Operating Procedures (SOPs)
- Common Technical Documents (CTD)
- Informed Consent, Lay Person Summaries and Patient Brochures
- Conference materials
- Educational materials and more…
Regulatory Submission
Regulatory Affairs is a large domain with the obligation of gathering and sending all types of data from development processes to regulatory bodies.
Pharmacovigilance Services
We cover drug and vaccine safety from Phase I to IV and throughout the development cycle, ensuring that ICH Good Clinical Practices are followed. We handle serious adverse effect (SAE) cases and produce quarterly reports, and we can help you with ongoing safety monitoring so you can keep track of your product's changing safety profile throughout the development process. We can collaborate with other CROs, various locations and investigators, and data safety monitoring board (DSMB) committees because of our flexibility.
Services:
- Safety database hosting including legacy case migration
- Global Literature Search & Review in support of the DSUR
- Medical Device Vigilance
- Medical monitoring and advice
- Reconciliation of Serious Adverse Events
- Development Safety Update Report (DSUR) preparation and submission
- Safety documentation storage and provision in eTMF format and more…
Decentralized Clinical Trial Solutions
A unique, highly secure, and scalable DCT technology lies at the heart of NextStage Decentralized Trials. All hybrid and fully virtual research activities can be orchestrated using the NextStage’s Decentralized Clinical Trials Platform.
- A track record of effectively deploying both hybrid and entirely virtual approaches.
- Collaborate across key stakeholders and systems
- Long-term follow-up studies in a virtual environment
- Technology specialists will advise on the best equipment, patient sensors, and applications to use in the study.
- Easily accessible staff maintaining open communication and more…
Clinical Endpoint Adjudication Process
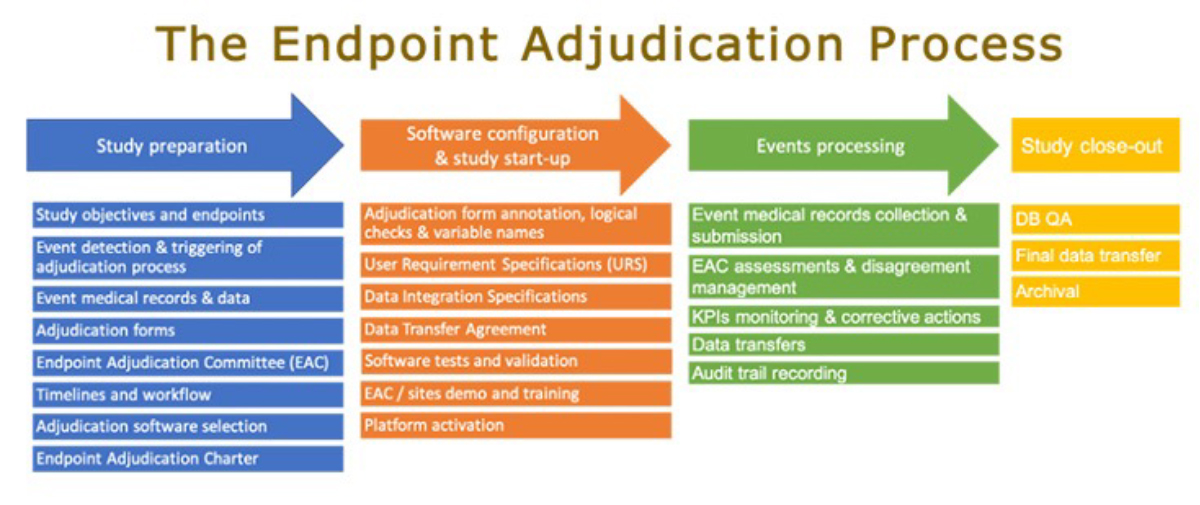
The clinical team decides and pilots the clinical adjudication process, but it requires input and assistance from numerous other teams, including operations, data management, and IT. The process might begin once the choice to adjudicate one or more clinical endpoints is made.
Phase 1: Study Preparation
Phase 2: Software Configuration and Study start-up
Phase 3: Events processing
Phase 4: Study close-out
Patient Recruitment
NextStage’s Direct-to-Patient Recruiting eliminates impediments that add time, cost, and uncertainty to recruitment efforts by combining unprecedented patient data and insight with machine learning. Our site enablement services supplement your trial recruiting efforts by allowing sites to focus on high-quality patient care, maximizing patient flow for greater efficiency, and continuously evaluating staff needs to reduce site staff strain.

and counting
and counting
Our network of physician groups represent a diverse range of patient populations and therapeutic areas to increase the power, effectiveness, and speed of clinical research innovation around the word. Together we represent more than 1.5 million patient charts, over 4 million patient visits annually, and over 70,000 surgical procedures across our rapidly growing network. Our physician partners all have access to surgical hospitals, surgery centers, or outpatient cardiac cath labs to provide options for patients and efficiency for sponsors.